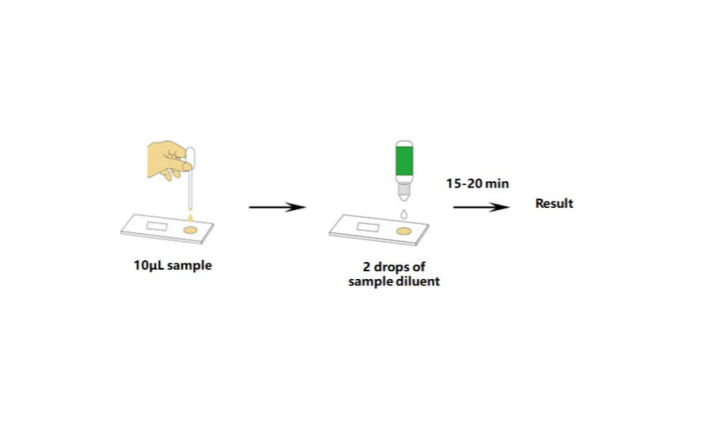
Covid 19 Antigen Test Eua - Covid-19 Realtime Info
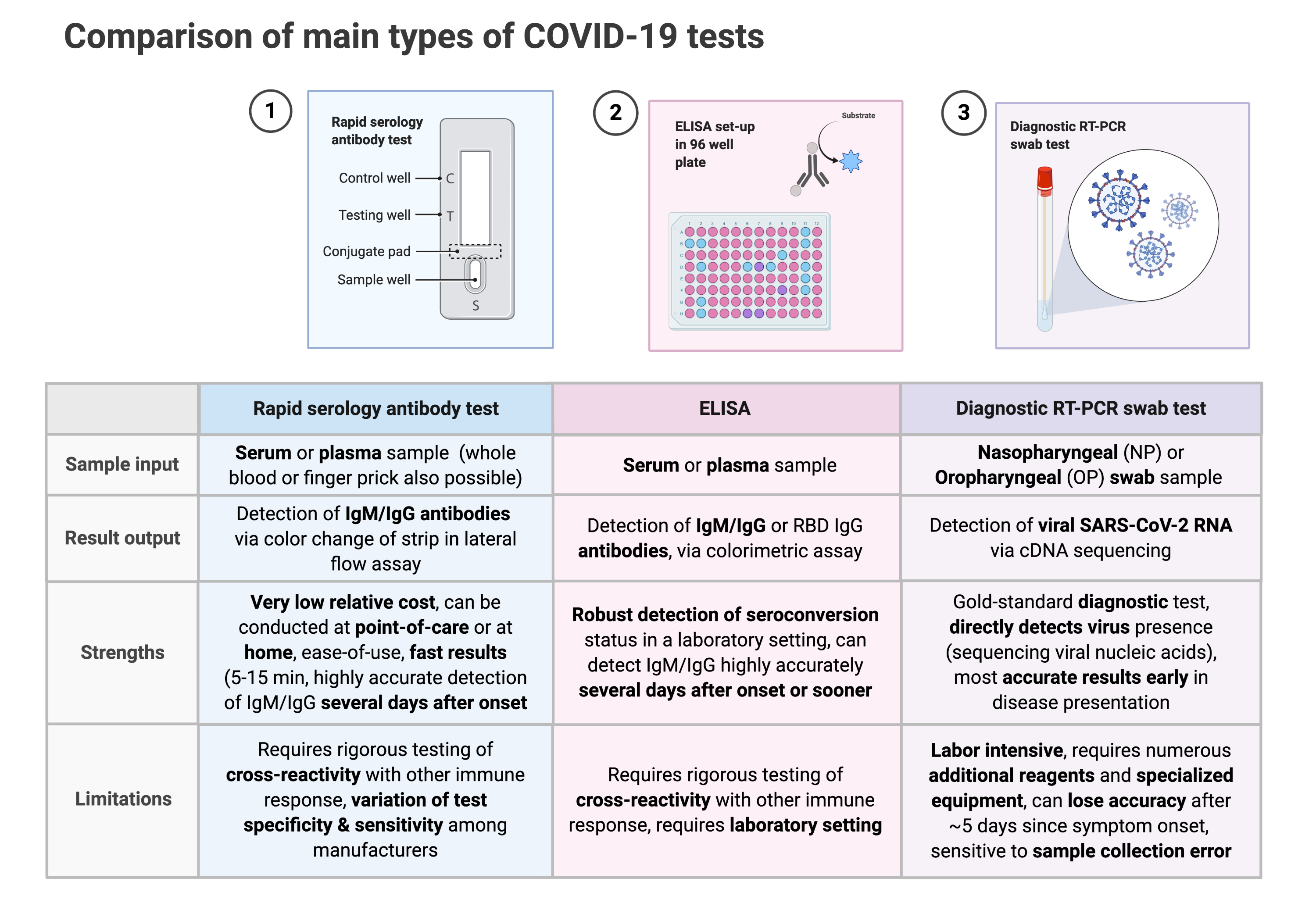
Fda granted the eua to the antigen test on the basis of a study of 257 nasal swabs taken from people with covid 19 symptoms and key workers.
![]()
Covid 19 antigen test eua. The results were the same most of the time giving the antigen test a specificity of 985 and sensitivity of 971. Abbott ran a study of 102 nasal swab samples taken from people within seven days of the onset of covid 19 symptoms to compare the results from its antigen test to the output of a pcr method. This new covid 19 antigen test is an important addition to available tests because the results can be read in. Specialty diagnostic sdi laboratories.
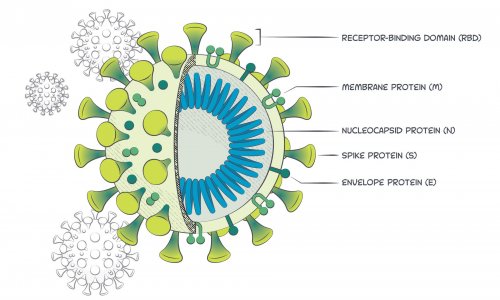
Template for manufacturers of molecular and antigen diagnostic covid 19 tests for non laboratory use. Fda has granted emergency use authorization eua to lumiradx uk ltd. Covid 19 antigen test receives eua similar in design to some pregnancy tests. That means the point of an antigen test is to detect the presence of a proteinthe nucleocapsid proteinwhich is part of the sars cov 2 virus that is the cause of covid 19 says dr.
Abbott has announced that the us. For its point of care sars cov 2 antigen test which aims to speed the diagnosis of people suspected of having the virus that causes covid 19. Antigen tests can be used in a variety of testing strategies to respond to the coronavirus disease 2019 covid 19 pandemic. Six facilities took the samples including four sites in which minimally trained operators collected and tested fresh specimens results from the lumiradx test were compared to a reference rt pcr assay.
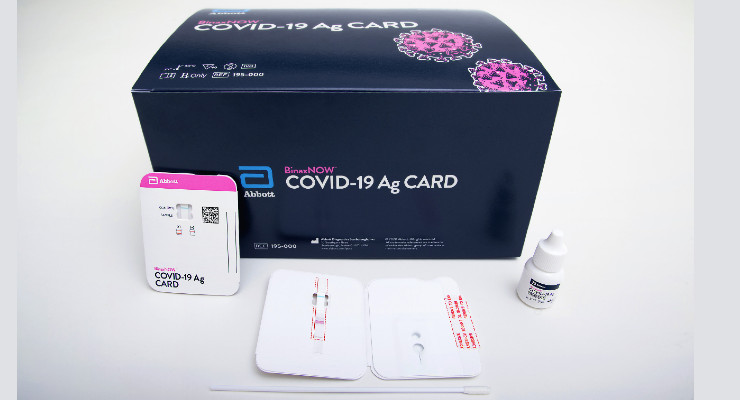
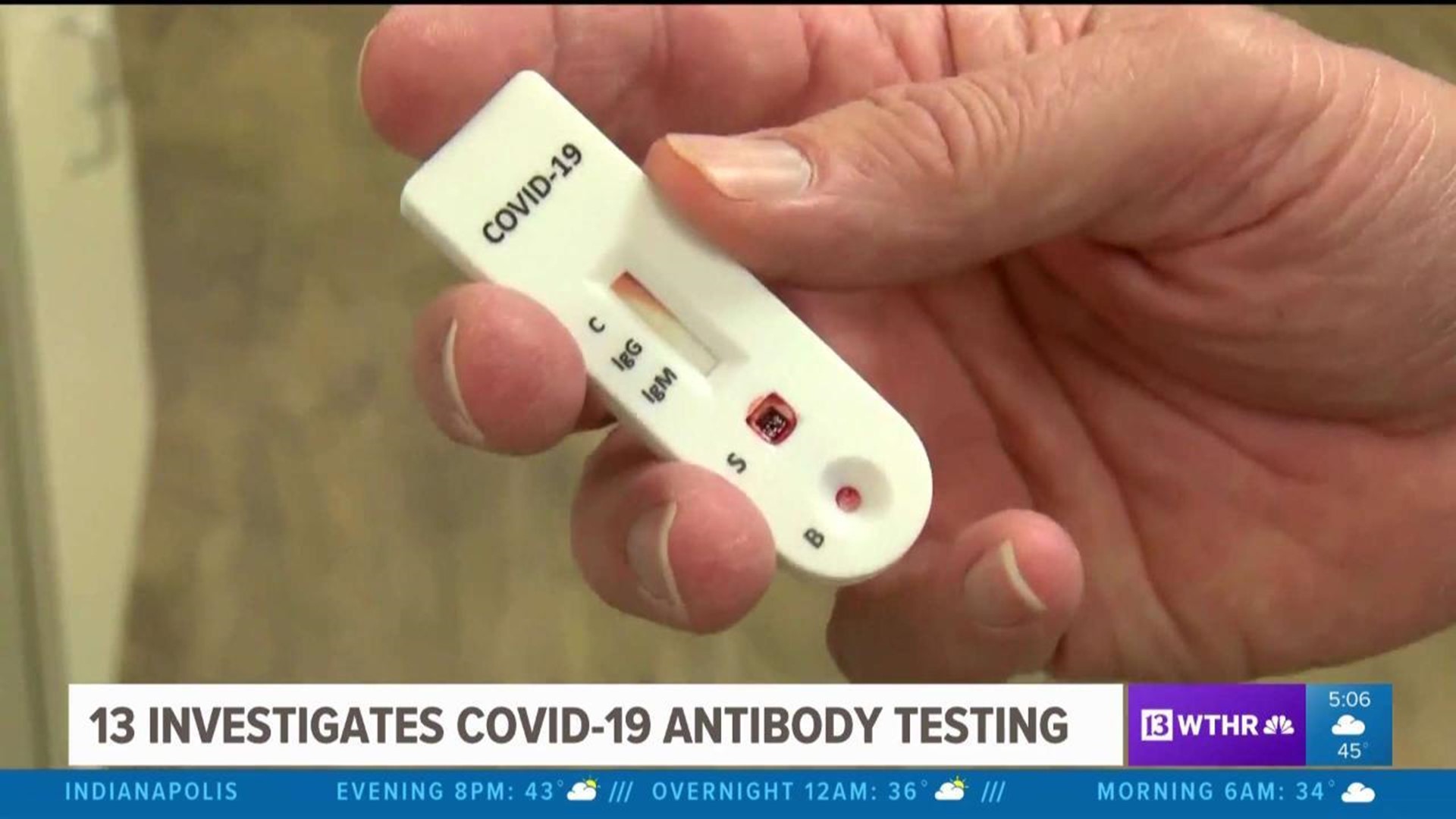
Becton dickinson bdx receives eua from the fda for a rapid point of care antigen test to detect sars cov 2 in 15 minutes. Food and drug administration fda has issued emergency use authorization eua for its binaxnow covid 19 ag card rapid test for detection of covid 19 infection. It is highly portable about the size of a credit card affordable and provides results in 15 minutes. Bd receives eua for new antigen test to combat covid 19 home.
This interim guidance is intended for clinicians who order antigen tests receive antigen test results andor perform point of care testing as well as for laboratory professionals who perform antigen testing in a laboratory setting or at the point of care and.